Introduction
Along with poultry and cattle, swine are among the species most susceptible to aflatoxins. Among the different types of mycotoxins, this group is positioned as one of the most toxic, being recognized for its high worldwide prevalence and its carcinogenic effects. Currently, more than 20 different types of aflatoxins are known, among which aflatoxin B1 (AFB1) stands out, recognized for generating harmful effects in both humans and animals (Popescu et al., 2022).
Aflatoxins can cause significant problems in pigs, either of an acute nature or due to chronic exposure. These compounds mainly exert toxicity at the hepatic level, with significant alterations in liver physiology and functionality (Pang et al., 2020). Prolonged exposure leads to states of immunosuppression, which have been associated with increased mortality, poorer performance, and reduced reproductive capacity (Popescu et al., 2022). In addition, their toxicity has been directly associated with greater susceptibility to respiratory pathogens and, consequently, a higher incidence of Porcine Respiratory Syndrome (Pang et al., 2020). AFB1 is also associated with toxic effects on the gastrointestinal tract, the nervous system, immune cells, and reproductive organs (Li et al., 2022).
On the other hand, it has been reported that the toxicity generated by aflatoxins is mainly associated with their metabolites. Specifically, the porcine species shows a lower detoxification capacity than other species, since its ability to excrete these metabolites is reduced, which increases its risk of intoxication and aflatoxicosis (Popescu et al., 2022). This detoxification encompasses a series of metabolic processes, which are studied through the science of toxicokinetics. The toxicokinetics of mycotoxins range from the absorption of these contaminants to their distribution in the organism, their metabolism, and finally their excretion. These processes are known as ADME processes, and their study is fundamental to understanding the toxicity of mycotoxins and to addressing how it varies among different animal species (Schrenk et al., 2020).
Another fundamental aspect related to the toxicokinetics of AFB1 in swine is that some metabolites of this mycotoxin have been detected in piglets, establishing the possibility of transfer through the placenta during gestation (Schrenk et al., 2020). Likewise, its transmission to milk has meant that the lactation process may be a source of exposure for newborns. On the other hand, this mycotoxin has been detected in other products for human consumption, such as meat and eggs. For this reason, exposure to feeds contaminated with aflatoxins not only poses a risk to animals, but also to the final consumer, constituting a food safety problem (Li et al., 2022).
BIŌNTE® QUIMITŌX® PLUS®: Toxicokinetic study of AFB1 in pigs
In this context, it is known that reducing oral bioavailability, defined as the fraction of a compound that can reach the bloodstream in an active form, constitutes a safe and efficient strategy to control mycotoxin toxicity. By controlling mycotoxins at the moment of absorption, their systemic distribution and that of their metabolites is reduced, decreasing the toxicity they can generate in different organs and their negative effects on animal health and performance.
In this case, through an in vivo toxicokinetic trial, carried out in collaboration with Ghent University, the effects of BIŌNTE® QUIMITŌX® PLUS on the oral bioavailability of AFB1 in pigs were evaluated, recording the plasma concentration profile of AFB1 as a function of time.
BIŌNTE® QUIMITŌX® PLUS® is an anti-mycotoxin solution that combines mineral ingredients for the selective and effective adsorption of these toxins, in addition to containing a specific mixture of natural extracts and selected yeasts that provide bioprotective and postbiotic effects. In this way, it constitutes a broad-spectrum option with multiple modes of action, efficient in controlling the negative effects of these contaminants in different terrestrial species.
The methodology used in this trial consisted of 8 female piglets, Belgian Landrace × Piétrain 7 weeks of age. These were divided into two experimental groups, and after a one-week adaptation period, a crossover design was established (Figure 1).
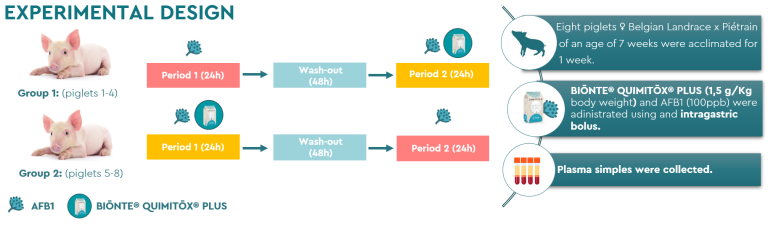
Figure 1. Experimental design.
Group 1 received, during the first period, an intragastric bolus of AFB1 (100 ppb), followed by a 48-hour washout period, and finally, during period 2, received another AFB1 bolus (100 ppb) accompanied by the product BIŌNTE® QUIMITŌX® PLUS®, at a dose of 1.5 g/kg body weight. On the other hand, group 2 received, during the first period, a bolus of AFB1 (100 ppb) together with the product BIŌNTE® QUIMITŌX® PLUS® at the same dose; this was followed by the washout phase, and in period 2 the animals again received a bolus of AFB1 (100 ppb).
Monitoring throughout the trial consisted of collecting blood samples both before the administration of the treatments and at controlled time intervals after administration.
The toxicokinetic analyses included the study of several parameters, such as the area under the plasma concentration–time curve from time 0 to 12 hours (AUC0→12), and the maximum plasma concentration (Cmax).
The effect of BIŌNTE® QUIMITŌX® PLUS on the oral absorption of AFB1 was evaluated by comparing toxicokinetic parameters based on plasma AFB1 concentrations in samples treated exclusively with AFB1 versus those from animals exposed to AFB1 together with the anti-mycotoxin product.
Trial results: Efficacy of BIŌNTE® QUIMITŌX® PLUS
Systemic exposure to AFB1 was significantly lower in those piglets that received AFB1 together with BIŌNTE® QUIMITŌX® PLUS, shown in yellow, compared to those that received AFB1 alone, shown in red (Figure 2). Specifically, AUC0→12 and Cmax showed reductions of 95% and 96%, respectively, due to the administration of the anti-mycotoxin solution (Table 1).
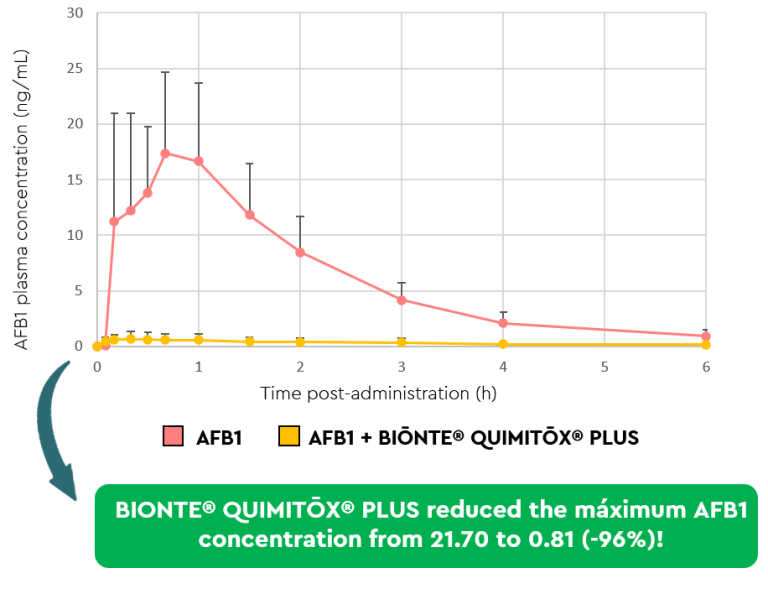
Figure 2. Plasma concentration–time profile of AFB1 following the administration of an oral bolus of AFB1 (100 ppb), alone or in combination with BIŌNTE® QUIMITŌX® PLUS®.
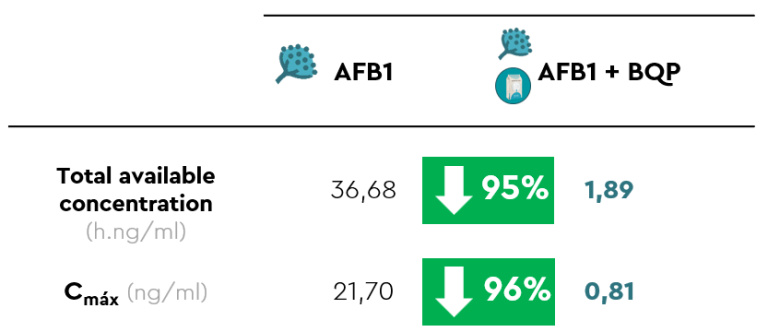
Table 1. Main toxicokinetic parameters of AFB1 after oral administration of AFB1, alone or in combination with BIŌNTE® QUIMITŌX® PLUS (BQP).
In addition to demonstrating its efficacy through the reduction of mycotoxin presence in blood, the results highlighted the rapid action of BIŌNTE® QUIMITŌX® PLUS, positioning it as a key strategy in mycotoxin control. These toxic compounds reach the bloodstream rapidly once animals have been exposed, and for this reason, efficacy and speed of action must go hand in hand when establishing effective strategies.
Conclusion
In conclusion, BIŌNTE® QUIMITŌX® PLUS proved to be highly effective, through this in vivo toxicokinetic study, in rapidly and efficiently reducing total systemic exposure in pigs exposed to one of the most well-known toxic mycotoxins, aflatoxin B1.


