Mycotoxins toxicokinetic is one of the fundamental aspects of mycotoxins exposure. Toxicokinetic refers to the relationship between the concentration of a substance (in this case the toxin) that an individual is exposed and the concentration of the active compound at the site of action (e.g. target organs such as the liver or kidney) where the toxins exert their effects (Johanson et al., 2010). The term absorption refers to the assessment of exposure at the site of action and more specifically to the rate of absorption (t max) from the site of application of the compound into the bloodstream (bioavailability, F). The term distribution indicates the rate and intensity of chemical transport from the blood to the tissues. The apparent volume of distribution (Vd) refers to the volume into which the amount of toxin is distributed to reach the same concentration in blood plasma. A further key mechanism in toxicokinetic is the metabolism, which provides information on the rate and extent of chemical biotransformation into water-soluble and inactive metabolites. Lastly, the term excretion refers to the elimination of the toxin from the body. During this process, the parameters of clearance (Cl: sum of all processes involved in the elimination of the toxin and its metabolites) half-life (t½: time required for plasma concentration of a substance to decrease by 50%) parameters provide information on the progression of the process.
As specified by EFSA, the acronym ADME indicates the four key processes describing how toxins enter the body, their metabolism and elimination “absorption, distribution, metabolism and excretion“, that allow us to understand the alterations that occur at the biochemical level, to apply diagnostic tests, to propose an appropriate treatment in cases of poisoning and to study the development and use of products. ADME processes are a necessary tool to evaluate the efficacy of anti-mycotoxins solutions.
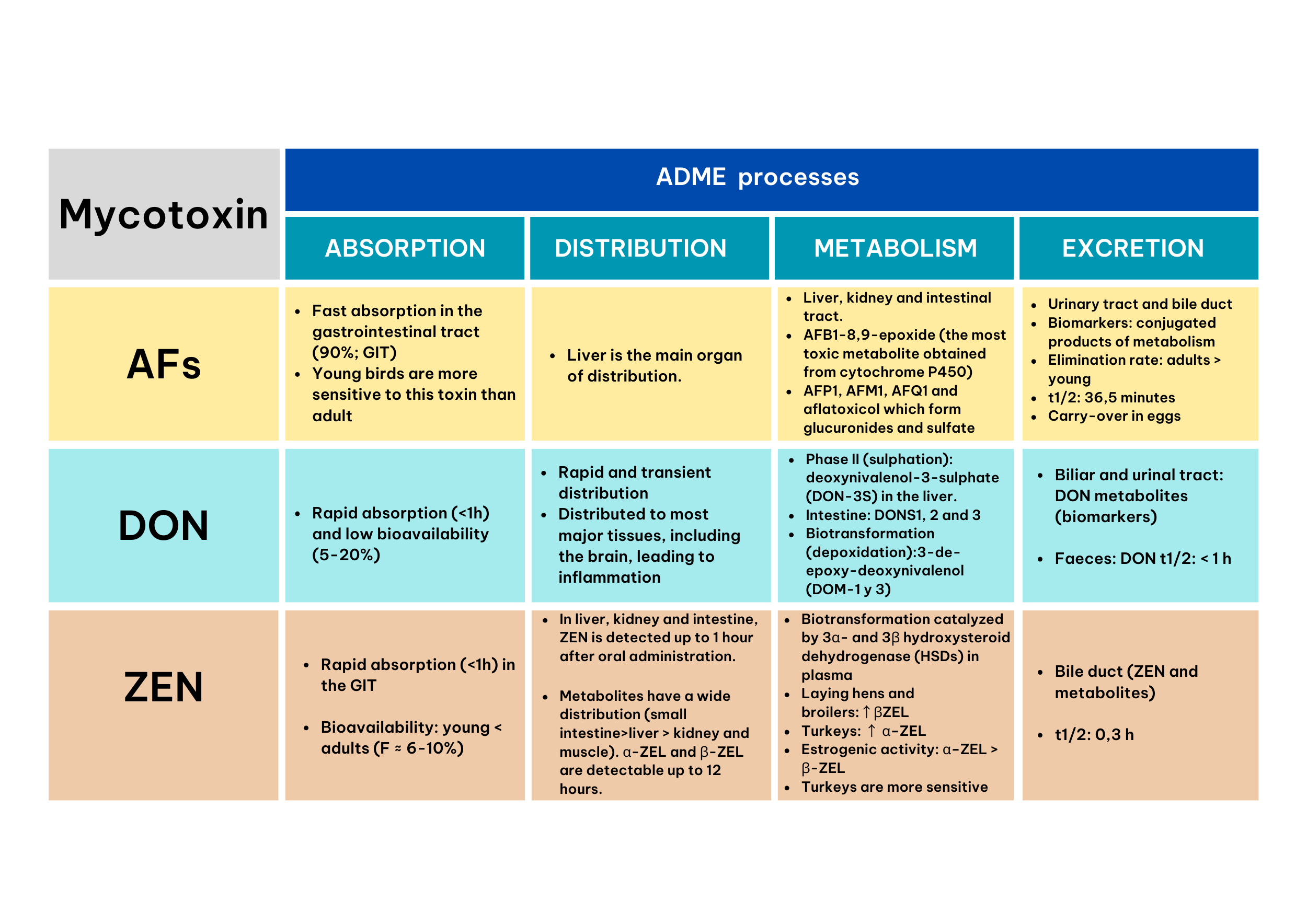
Aflatoxins (AFs)
Absorption
Aflatoxins mainly AFB1 are highly absorbed in the gastrointestinal tract of poultry (90%) and are well known for their carcinogenic effect which can affect any organ or system, especially the liver and kidney.
Distribution
The target site of AFs is the liver (Schrenk et.al, 2020). Poultry with aflatoxicosis showed an impairment in the fatty acid composition at the hepatic level, resulting in extended lipid peroxidation, and thus to the generation of oxidative stress and decreased enzymatic and non-enzymatic antioxidants production (Allah et. al, 2018).
Metabolism
Biotransformation of aflatoxins takes place in the liver, kidney and intestinal tract. The biotransformation products are less toxic than AFB1, except AFB1-8,9-epoxide, which is obtained from cytochrome P450 pathway. Others produced metabolites are AFP1, AFM1, AFQ1 and aflatoxicol which form glucuronides and sulfate conjugates. Among poultry species, turkeys and young animals are the most sensitive to AFB1 (Coppock et.al, 2018).
Excretion
Aflatoxins are excreted via urine, eggs, bile and feces (Coppock et.al, 2018). The elimination of AFB1 from tissues has been shown to be faster in adult animals than in young animals (Hussain et.al, 2010). The plasma half-life is 36.5 minutes (Allah et.al, 2018).
Deoxynivalenol (DON)
Absorption
The absorption of this mycotoxin is rapid (Schrenk et al., 2023), with a maximum absorption time of 1 h (Sun et al., 2022). In poultry, DON bioavailability is low and ranges from 5% to 20% (Schrenk et al., 2023).
Distribution
In poultry, DON is widely distributed in many tissues (Schrenk et.al, 2023). Its distribution is rapid and transient in tissues such as: serum, muscle, abdominal fat, stomach, intestine, liver, kidneys, heart, brain, lung, skin, spleen, testes, ovaries and adrenal glands (Devreese et al., 2015).
Metabolism
Sulfation and de-epoxidation represent the main metabolic pathways of this mycotoxin, resulting in less or non-toxic metabolites. An extensive sulfation is observed in the liver and intestine, with the predominant metabolite being DON 3-sulphate (DON-3S). In addition, efficient de-epoxidation of DON to DOM-1 and DOM-3 takes place in the intestine, which has less strong effects on poultry. Other intestinal metabolites include DONS1, 2 and 3-sulphonates (Schwartz et al., 2015).
Excretion
This mycotoxin is rapidly excreted from the body. Metabolites of DON are excreted via bile, urine and a small amount of unmodified DON can be found in feces and present an average half-life of less than 1 h (Schrenk et.al, 2023).
Zearalenone (ZEN)
Absorption
This mycotoxin is rapidly absorbed in chickens, laying hens and turkeys, usually between 5 minutes and 2 h (Liu and Applegate, 2020). Some authors have reported low bioavailability in younger animals (Liu and Applegate, 2020). In addition, Devreese et al. (2015) observed a bioavailability of 6.87% to 10.2%.
Distribution
The metabolites of ZEN, α-ZEL and β-ZEL, can be detected in several tissues and body fluids, including blood, liver, kidney, muscle, intestine and faeces (Buranatragool et al., 2015). After oral administration, ZEN can be found in the liver, kidney and small intestine within 1 h after ingestion, while α-ZEL and β-ZEL remain detectable in these same organs for a maximum of 12 h (Devreese et al., 2015). In addition, both ZEN and its metabolites can be found in muscle until 1 h after exposure (Devreese et al., 2015).
Metabolism
In broilers, ZEN is rapidly converted to α-ZEL and β-ZEL in plasma. Laying hens and broilers show a dominant biotransformation of ZEN to β-ZEL, whereas, in turkeys, ZEN is preferentially converted to α-ZEL and show a higher sensitivity (Liu and Applegate, 2020).
Excretion
In poultry, the bile has been identified as a major route of excretion for ZEN and its metabolites, suggesting that the main route of elimination is via the feces. In addition, studies in broilers, turkeys and laying hens estimated that the half-life of ZEN is approximately 0.3 h (Knutsen et al., 2021).
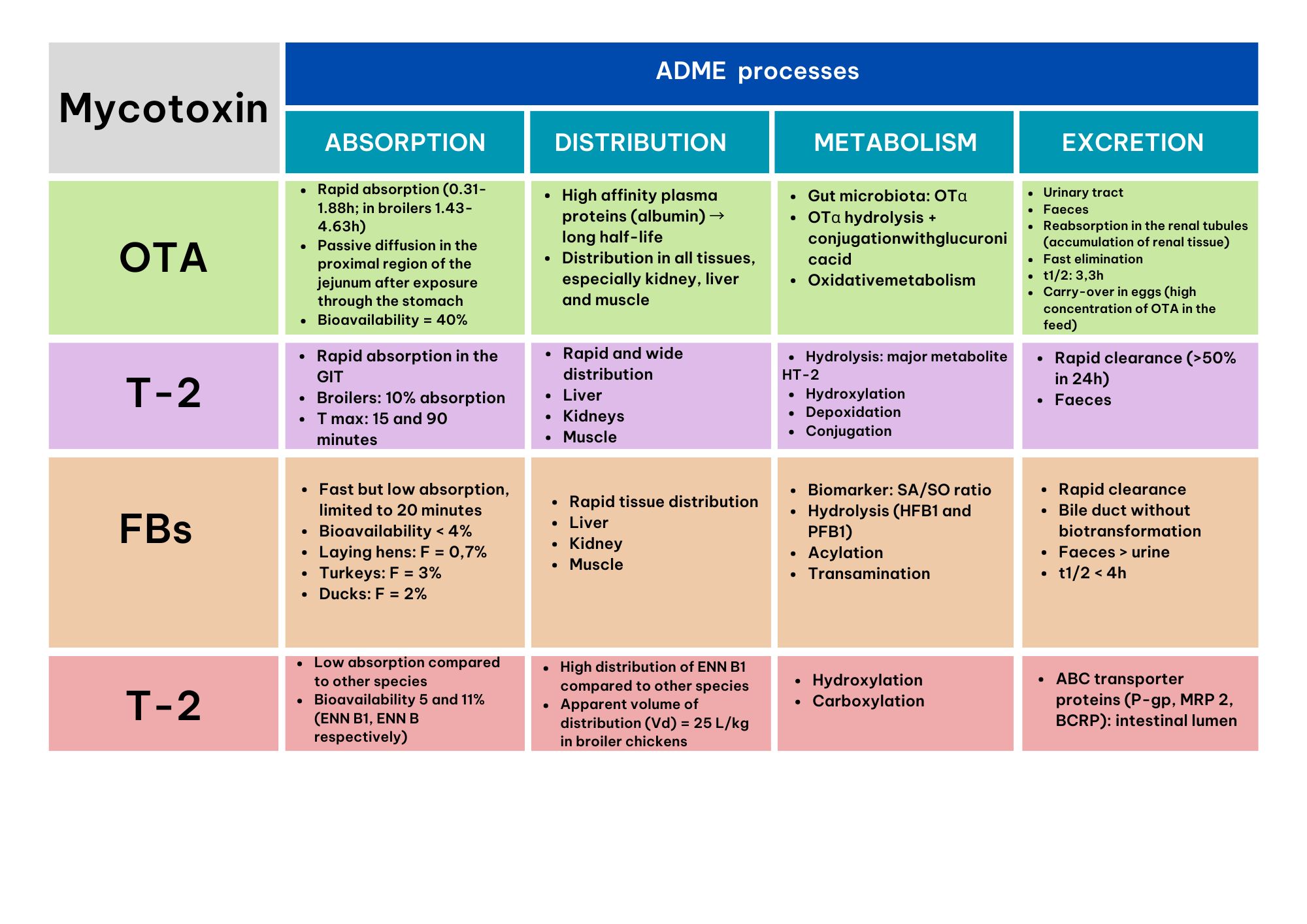
Ochratoxin A (OTA)
Absorption
Poultry show rapid absorption and a high concentration of OTA in the blood over a period of several hours. This process occurs mainly through passive diffusion, particularly in the proximal region of the jejunum, after exposure through the stomach. In chickens, OTA bioavailability is estimated to be around 40% (Schrenk et al., 2020). In a study by Devreese et al. (2018), OTA absorption was observed to be rapid, with a maximum time of 0.31 and 1.88 h in laying hens, turkeys and ducks. In broiler chickens, this maximum time extends to 1.43-4.63 h.
Distribution
OTA is distributed in all organs. Kidney, liver and muscle are the organs with most residues of this mycotoxin (Shrenk et.al, 2020).
Metabolism
The main metabolite of OTA is OTalpha (OTα), which is metabolized by the intestinal microbiota in monogastric animals. OTα is partially absorbed in the gut, but does not accumulate in the kidney; instead, it is rapidly excreted in the urine as a glucuronide. The predominant metabolic pathway involves hydrolysis of OTα followed by its conjugation with glucuronic acid, with oxidative metabolism being of minor relevance (Schrenk et al., 2020).
Excretion
OTA is slowly cleared and excreted due to binding to plasma proteins which have a low rate of metabolism. Furthermore, it has been reported that there is a reabsorption of secreted and filtered OTA by the kidney leading to an accumulation in renal tissue, delaying its excretion (Shrenk et. al, 2020). The excretion process of OTA and its metabolites takes place via urine and feces. Poultry eliminate OTA faster than monogastric animals, resulting in a lower accumulation of OTA. Transfer of OTA to the egg occurs when its concentration in the feed is high, such as 10 mg/kg per kg of live weight (Schiavone et. al, 2008; Battacone et. al, 2010). Ruprich et.al (1991) found a half-life of 3.3h.
T-2 toxin
Absorption
T-2 toxin, like other trichothecenes, is rapidly absorbed in the intestinal tract, and the 80-90% of it is metabolized and eliminated within 48 h (Sokolovic et al., 2008). Broekaert et al. (2014) reported an absorption rate of 10.6% only in broiler chickens after administration of 0.5 mg/kg per kg of live weight. Maximum absorption of T-2 is observed at 15 and 90 minutes after consumption of contaminated feed (Reddy et al., 2004).
Distribution
T-2 toxin is widely and rapidly distributed as in other species. The peak concentrations of T-2 toxin and its metabolites were observed at 3 h in liver and kidneys and at 4-6 h in liver, muscle and kidneys (Chi et al., 1978).
Metabolism
T-2 toxin is generally metabolized and eliminated after ingestion, with more than 20 metabolites (HT-2 is the principal one). The main metabolic reactions are: hydrolysis, hydroxylation, depoxidation and conjugation (CONTAM, 2011).
Excretion
This mycotoxin is rapidly eliminated (more than 50% in 24 h) via feces (CONTAM, 2011).
Fumonisins (FBs)
Absorption
The FBs absorption is around 2-3% and mainly occurs in the gastrointestinal tract (Knutsen et.al, 2018). Absorption is rapid, but limited to 20 minutes after feed administration (Knutsen et.al, 2018). Turkeys and ducks are more sensitive to these mycotoxins than laying and breeding hens.
Distribution
It is rapidly distributed in tissues and is mainly found in liver, kidney and muscle (Knutsen, et al., 2018).
Metabolism
The metabolism of FBs includes hydrolysis reactions (the hydrolyzed, HFB1 and partially hydrolyzed forms, PFB1), acylation and transamination in the liver (Hartinger et al., 2011). Given the high structural similarity to sphingolipids, the alteration of the sphinganine/sphingosine ratio is considered a biomarker of great interest to determine the toxicity of fumonisins (Guerre et al., 2022).
Excretion
FBs are excreted via biliary pathway without biotransformation and have a higher elimination in feces (usually 90% of the dose) than by the urinary route. Both FBs and their metabolites have been detected in feces, with a half-life of less than 4h (Knutsen, et al., 2018).
Emerging Mycotoxins
Emerging mycotoxins refers to a group of toxins that differ in structure from those mentioned above and for which, to date, there is no regulation, and include fusaproliferin, beauvericin, enniatins and moniliformin, all produced by the genus Fusarium (Krska et al., 2015).
Enniatins (ENN) and Beauvericin (BEA)
Absorption
In poultry, emerging mycotoxins are poorly absorbed compared to other species, with a bioavailability (F) of 5% and 11% for ENN B1 and B, respectively.
Distribution
ENN B1 has a high tissue distribution in chickens with a volume of distribution of 25 L/kg in broiler chickens (Fraeyman et al., 2016).
Metabolism
The metabolism of these mycotoxins includes hydroxylation and carboxylation reactions (Ivanova et al., 2011).
Excretion
These mycotoxins are eliminated by ABC transport proteins (P-gp, MRP 2, BCRP) directly to the intestinal lumen. A high elimination has been described in chickens compared to other species. Furthermore, it must be considered that the low bioavailability and high elimination of enniantins in poultry result in a low toxicity in this species.
Conclusions
The study of the toxicokinetic process allows to the knowledge of the susceptibility of target species and their different categories to different mycotoxins in animal nutrition.
Table 1. Toxicokinetics of common and emerging mycotoxins in animal production